How does flux residue influence condensation and failure?
By Abram Agaiby
The combination of flux residue and condensation creates a perfect environment for electrochemical reactions. This is why using proven, reliable cleaning or low-residue/no-clean fluxes under harsh conditions is critical.
Many flux residues are hygroscopic, meaning they attract and absorb moisture from the environment. When condensation occurs, hygroscopic flux residues can trap water on the PCB surface.
Moisture combined with ionic contaminants in flux residue creates an electrolyte layer. Flux residues often contain weak organic acids and halides. In the presence of moisture, these chemicals accelerate the corrosion of metal finishes and solder alloys, degrading solder joints and traces.
Figure 1: Ionic Contaminants attract Moisture
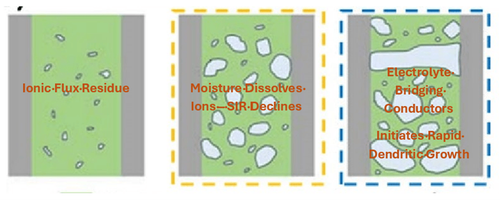
The presence of moisture and ionic residues increases surface conductivity. Moisture absorption lowers SIR because water is a polar molecule that can dissolve ionic residues and create conductive pathways next to soldered pads of opposite polarity. If flux residues or other ionic contaminants are present, moisture acts as a solvent. Dissolved ions become mobile and form an electrolyte layer in water, which reduces insulation resistance.
When the device is powered on, the bias voltage attracts positively charged ions in the moisture layer, allowing the metal to migrate and form dendrites. This accelerates the decline in SIR and leads to eventual shorts.
Take Away: Moisture doesn’t just add water – it activates contaminants and creates a conductive medium, which is why SIR drops.